Tesomet
Tesofensine + metoprolol
Tesomet is an investigational fixed-dose combination therapy of tesofensine (a triple monoamine reuptake inhibitor) and metoprolol (a beta-1 selective blocker). Saniona’s Phase 2b trials of Tesomet in hypothalamic obesity and Prader-Willi syndrome have been voluntarily paused due to funding limitations; this pause is not related to the safety or efficacy of Tesomet. Saniona holds worldwide rights to Tesomet and is actively evaluating opportunities to advance this treatment globally.
How Tesomet works
TESOMET
fixed-dose combination
of tesofensine + metoprolol
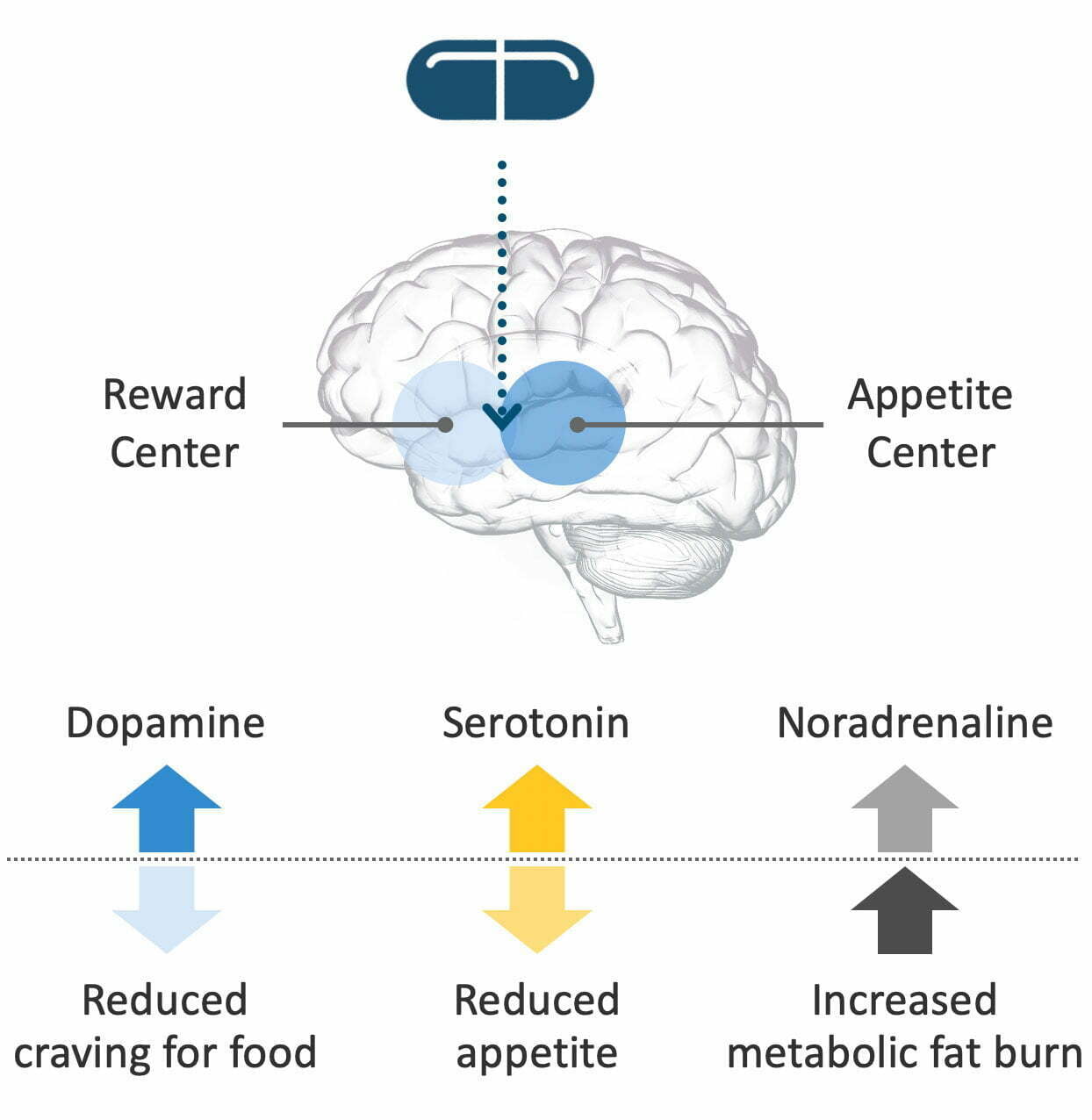
Tesomet combines two active ingredients: tesofensine and metoprolol. Tesofensine is a novel, proprietary molecule developed in the labs of Saniona’s founding scientists. It works in the brain to block reabsorption of three monoamine neurotransmitters: serotonin, noradrenaline and dopamine. Blocking reuptake increases the level of these neurotransmitters in the brain, which reduces cravings for food, reduces appetite, and increases metabolic fat burn.
When administered alone, tesofensine has been shown in clinical studies to reduce weight while being generally well-tolerated. However, some doses resulted in an increase in heart rate and/or blood pressure. Tesomet combines tesofensine with metoprolol, a generic medicine that is used to treat hypertension, angina pectoris and heart failure. The Tesomet combination has demonstrated a significantly improved cardiovascular safety profile compared to tesofensine alone.
TESOMET STATUS
Saniona’s Phase 2b trials of Tesomet in hypothalamic obesity and Prader-Willi syndrome have been voluntarily paused due to funding limitations; this pause is not related to the safety or efficacy of Tesomet.